Bringing to the table twenty years of market experience, Neutralox offers expertise in providing high quality physical-chemical treatment solutions. The Neutralox Photoionization process is not sensitive to environmental influences and provides high treatment efficiency, operational reliability, and low O&M demand. Their years of experience are put to work assisting clients not only with equipment, but also with operational insight and design guidance from the beginning of a project.
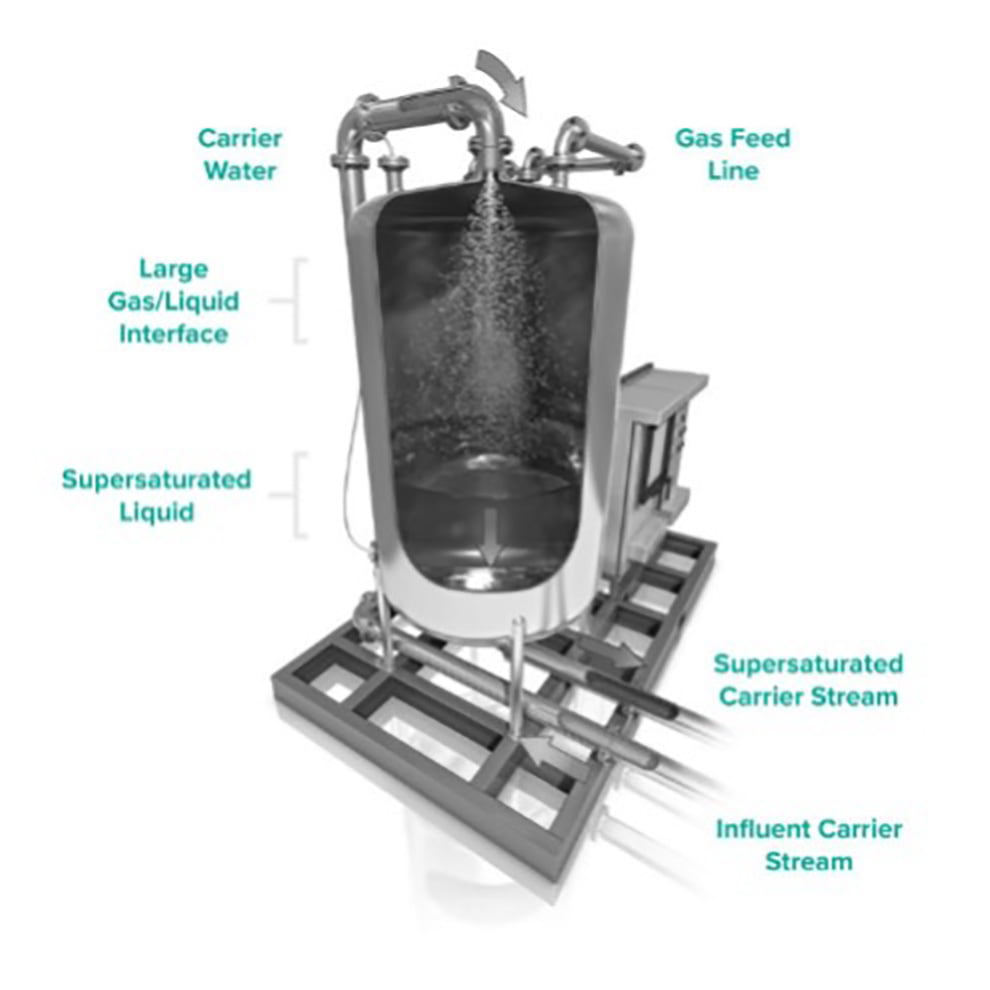
Combat odour and corrosion problems in your collection system with BlueInGreen technology. The SDOX® utilizes a pressurized process to rapidly and efficiently dissolve oxygen in a side stream and is designed to eliminate sulfide production by promoting an aerobic environment. BlueInGreen’s odour control solutions offer an effective alternative to chemical treatment in force mains and gravity sewers, combining greater control and precision with a lower life-cycle cost.
Applications Include: H2S Reduction, Dissolved Sulfide Removal, Corrosion Control, Chemical Replacement


Biofiltration – The Ambio Way
Biofiltration is an air pollution control technology made up microorganisms grown on a packed bed of support media. The contaminated air stream, with pollutants such as: ammonia, amines, hydrogen sulfide, reduced sulphur compounds, and/or volatile organic compounds, is humidified and passed through the bed. This technology requires minimal energy and raw materials and produces minimal waste. Biofiltration has been successfully used in a variety of odour control applications in North America including wastewater treatment plants, food processing plants, composting & organics processing facilities, rendering plants, fertilizer plants, pulp & paper industries, ATAD off gas treatment and VOC emission control (BTEX control).
Biofiltration – “The Ambio Way” is the embodiment of Ambio Biofiltration’s almost 30 years of experience in the design of simple and cost effective biofiltration odour control systems. Our odour control systems are regularly smaller, have a lower cost per volume of air, and fewer maintenance requirements than our competitors.